The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) is the first significant update to the Food and Drug Administration's (FDA) cosmetics regulations since 1938, and comes into force this year. This new law is designed to protect the safety of cosmetic products that many consumers use on a daily basis and to bring U.S. cosmetics regulations more in line with the EU cosmetics regulation.
The new law introduces significant changes to cosmetics regulations int the U.S. such as the new concept of a “Responsible Person” (RP), new labelling requirements, mandatory product listing and facility registration, reporting of serious adverse events, and finished product safety assessment. The regulation will also establish a new Good Manufacturing Practice (GMP) standard for cosmetic products manufactured in the U.S.
Any product that fails to comply with the new requirements will be prohibited, so it is imperative that businesses affected by this law start preparing for their products to meet the requirements.
Certification to the BRCGS Consumer Products (Personal Care and Household) Global Standard Issue 4 will help to ensure that any site either selling cosmetic products in the USA or exporting to the USA will comply with the act.
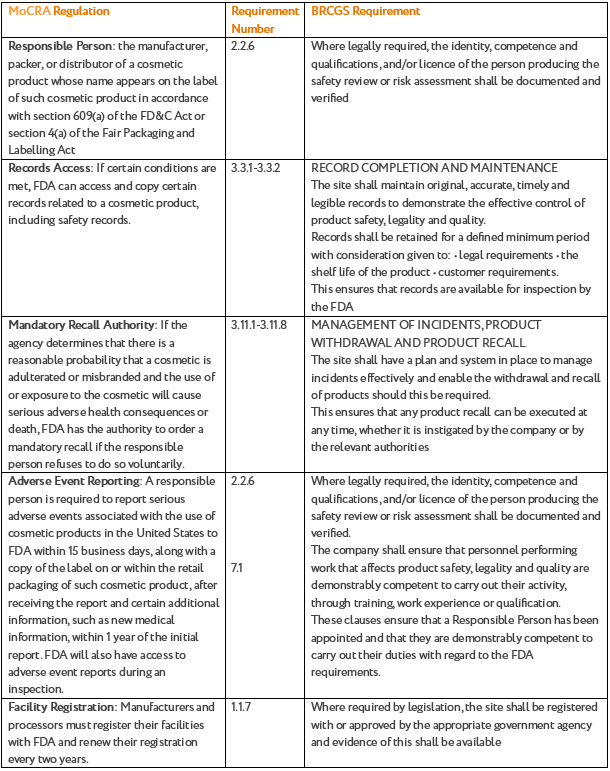
Section 2.1 of the BRCGS Standard states that the site shall have a system to demonstrate knowledge of all applicable legislation, product standards and safety issues in the place of production and regions of intended sale. Below we have provided a comparison table to map out the requirements:
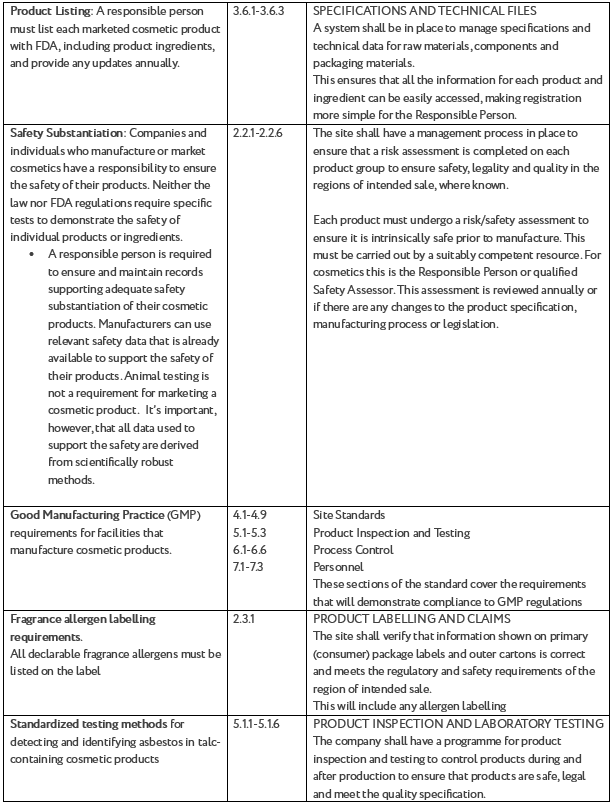
|
|
Certification to the BRCGS Consumer Products (Personal Care and Household) Global Standard will help to ensure that a site will comply with MoCRA with regards to legislative, recordkeeping, traceability, product recall and GMP regulatory requirement.
For further information please visit the BRCGS website or contact us at brcgs.enquiries@lgcgroup.com if you have any questions.